New Neural Pathways
Revolutionizing immunotherapy with a non-invasive moonshot for Nesos
Nesos’ novel therapeutic device delivered powerful results in early clinical testing. But, what would it take to inspire trust and adherence with patients outside the lab?
Our insights propelled Nesos forward with confidence and with strategic opportunities to establish trust, effectively deliver therapy, and inspire adherence in their first product.
- Generative Research
- Competitive Analysis
- Literature Review
- Clinical Data Analysis
- Analogous Research
- Contextual Inquiry
- Participatory Design
- Formative Research
- Experience Strategy
- Design Strategy
- Market Access Strategy
Foundation

To probe into new technologies and unfamiliar territory, prototyping a discovery experience is critical.
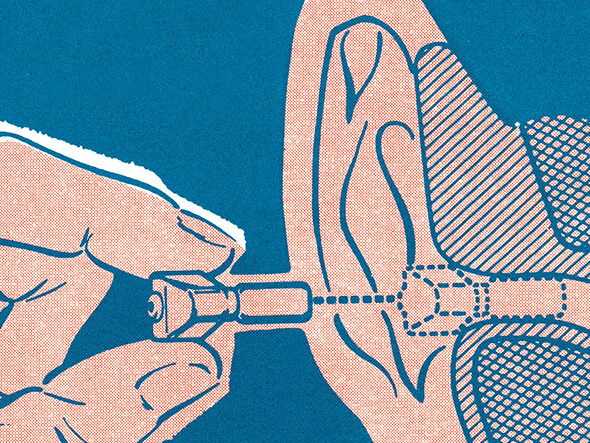
Nesos’ innovation, a non-invasive, wearable, therapeutic medical device, demonstrated remarkable clinical results for people suffering from a range of conditions regulated by the immune system – from rheumatoid arthritis to migraine and postpartum depression. Where neuroscience and immunology were once conceived of as entirely discrete disciplines, modern science has now proven the strong link between them: the brain-gut connection. This renders the brain critical in targeting inflammation and healing. By sending electrical signals to the brain to redefine its neural pathways for activating the body’s own capacity to heal, Nesos’ non-invasive therapy certainly sounds like a “moonshot.” The potential disruption to the current pharmaceutical paradigm is profound. With a proof-of-concept and strong clinical research, the missing piece was the people quotient. Would patients be receptive to this emerging solution? How might the product design and experience create trust and confidence in this new therapy?
Research
From the lens of desirability, we created a dynamic discovery program to understand the appetite, barriers, unmet needs, and perceptions of people managing their health with conditions that Nesos’ therapy could improve. Through a carefully sequenced mixed methods study comprised of inspiration activities, auto-ethnographic inputs, competitive landscape analysis, and product audits, we prototyped our way through an immersive experience with our client team, and a highly informative and deeply moving project.


In addition to our core indication areas, we studied expert users in the health, sports, and wellness domains for a robust study of 40 participants, identifying signals from early adopters and power users that can teach us which behaviors and mindsets most contribute to success and satisfaction.




Contextual inquiry, interactive co-design activities, product comparisons, ratings, and anchoring techniques unveiled critical unmet needs and expectations that revealed our must-deliver requirements.
Selected Insights
- Clinical Authority: A Double-Edged Sword
Clinical condescension and skepticism of traditional clinicians and their relationship with the pharmaceutical industry emerged as prominent themes in our exploration of non-traditional, non-invasive therapies, signaling high receptivity and actionable design opportunities.
- Pain & Prejudice
To the naive observer, conditions like rheumatoid arthritis, migraines, and postpartum depression are invisible conditions. They are also conditions which skew predominantly female – about 80%. Women and people of color are especially targeted, harassed, and dismissed in voicing concern about symptoms and treatment.
We discovered heart-wrenching stories of clinicians accusing women with migraines as “crazy” and insisting their need for pain relief was because they were “drug addicts,” and Black rheumatoid arthritis sufferers of “trying to get high” by using topical CBD to soothe the pain in their inflamed hand joints. One Black woman was even dismissed from her local ER when she felt something off during her pregnancy. She traveled to a second, out-of-network hospital that listened to her, and imaging revealed her baby’s umbilical cord wrapped around its neck in utero. They performed an emergency C-section and both mother and child survived.
- #BelieveThem
The inability to listen to and empathize with patients costs lives – and erodes trust. The inherent wisdom of the body should be a priority for clinicians, but when it doesn’t fit into protocol, it’s not. Vulnerability in all its forms is still stigmatized in our society.
Outcomes

Contextual inquiry, interactive co-design activities, product comparisons, ratings, and anchoring techniques unveiled critical unmet needs and expectations that revealed our must-deliver requirements.
Our methods enabled us to assess desirability for an at-home therapeutic, and to determine the cognitive and emotional resonance with this new therapy. We uncovered the individual and cultural barriers that need to be overcome for this product to be introduced successfully, used regularly, and integrated into real people’s everyday lives.
Through our highly effective comparative anchoring method, we determined price and insurance thresholds that informed cost and viability. Thus, we paved a path to market access with pricing, merchandising, and partnership recommendations to position the product for maximum adoption and treatment at scale. We further built a pathway for the service and experience design strategy, along with marketing and advertising messaging. Nesos has just secured over $15m in funding, completed more affirming clinical research, and is pursuing FDA approval for this device to treat rheumatoid arthritis, with more conditions likely to be approved in the years ahead.